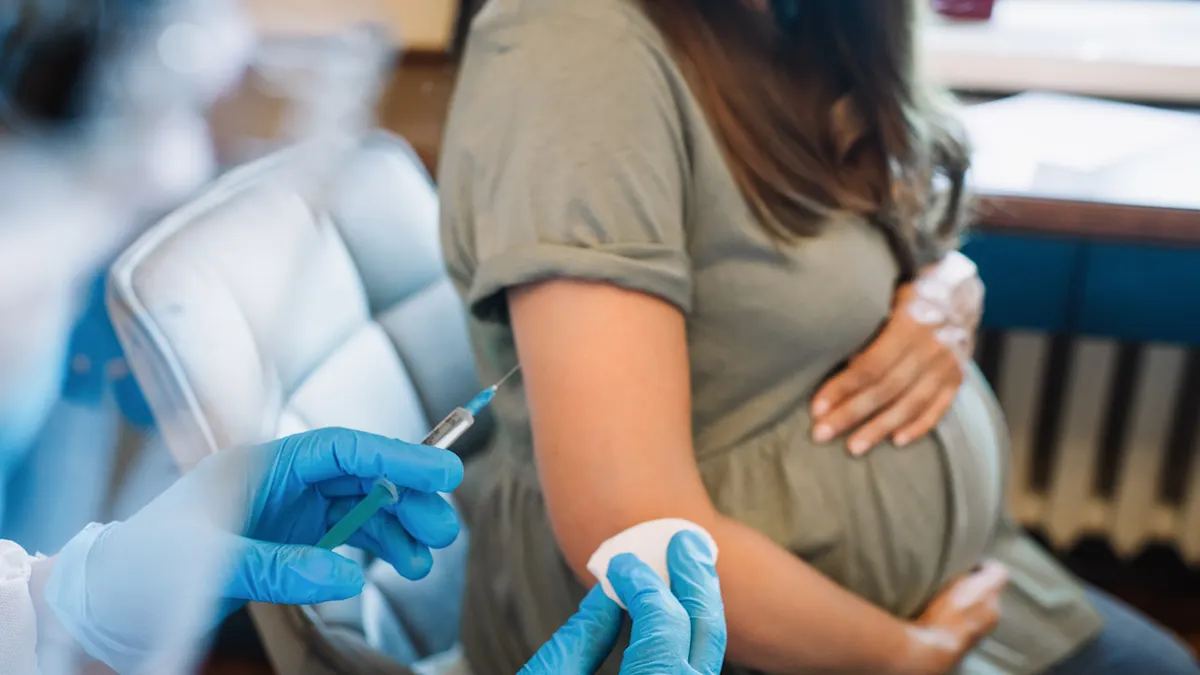
Respiratory Syncytial Virus (RSV) is the leading cause of infant hospitalization in the United States, and its effects can be devastating. Severe RSV infections lead to approximately 80,000 annual hospitalizations among children under five, with a significant number facing complications like intubation or chronic respiratory diseases. In fact, RSV is responsible for 3.6% of infant deaths worldwide, particularly those aged 28 days to six months. For years, researchers and healthcare providers have been striving to develop solutions to protect vulnerable infants from this potentially life-threatening disease. Recent studies have brought a ray of hope for pregnant women, suggesting that a new RSV vaccine for pregnant individuals can protect newborns from severe RSV while being safe for both mothers and babies.
The RSV Vaccine: A Promising Breakthrough
One of the biggest concerns around any vaccination, particularly for pregnant individuals, is whether it poses risks to the pregnancy itself. Prior RSV vaccine trials raised concerns about the potential risk of preterm birth, which has caused hesitation in some expecting mothers when considering immunization during pregnancy. However, the latest studies indicate that the RSV vaccine given between the 32nd and 36th week of pregnancy does not appear to be associated with preterm birth or other pregnancy complications.
Dr. Christine Blauvelt, a maternal-fetal medicine fellow at UCSF Health, co-authored one of the studies and offered reassuring words about the findings. According to Blauvelt, the data from their study revealed no increased risk of pregnancy complications, including preterm birth, among those vaccinated during pregnancy. She emphasized the safety of the vaccine and highlighted that a significant number of babies received protection against RSV through both the vaccine and a monoclonal antibody treatment called nirsevimab. This two-pronged approach showed promising results, with up to 80% of babies protected for the 2023-24 RSV season.
This data provides hope for widespread immunity against RSV, which has historically caused devastating effects on infants. Furthermore, it suggests that the combination of vaccination during pregnancy and the use of monoclonal antibodies may provide population-level prevention, drastically reducing the transmission of RSV and protecting infants at their most vulnerable stages of life.
High Uptake and Reassuring Results
In the new study conducted by UCSF Health, approximately 64% of pregnant individuals received the RSV vaccine before delivery, and about 70% of eligible newborns received the nirsevimab treatment. These combined efforts contributed to an impressive 80% protection rate against RSV during the 2023-24 season. The study’s results were encouraging, demonstrating high uptake rates and reassuring perinatal outcomes for both mothers and babies.
Not only did the study show no adverse pregnancy outcomes, but it also provided important insights into the potential for creating herd immunity in communities with high vaccination and antibody treatment uptake. In some areas, the protection rate among babies was so high that it could offer a means of stopping RSV from spreading on a larger scale. The study’s findings were published in JAMA Network Open, offering hope that widespread vaccination efforts could make RSV a preventable disease in the near future.
A Global Perspective
While the success of these studies offers hope, RSV immunizations still face challenges. One such hurdle is vaccine hesitancy, which was particularly evident in the 2023 rollout of RSV immunizations. Despite the clear benefits, some pregnant individuals opted for monoclonal antibody treatments over the RSV vaccine, especially in communities such as California. Although monoclonal antibodies like nirsevimab are not technically vaccines, they are still a vital tool in reducing RSV infections. These treatments help protect newborns from RSV for the first few months of life, when the risk of severe complications is highest.
In California, where the new vaccine was first offered to pregnant individuals, mothers who declined the RSV vaccine often chose monoclonal antibody treatment. According to Blauvelt, this approach is also effective and provides a vital alternative for those who may have concerns about receiving the vaccine during pregnancy. “We know that infant RSV infections can lead to serious complications including hospitalization, intubation, and chronic respiratory disease,” Blauvelt said. “Prenatal RSV vaccination and infant monoclonal antibodies against RSV are safe and effective ways to protect babies against RSV during the first 6 months of life when they are most vulnerable to this disease.”
Logistics and Access to Vaccination
Another critical point raised by the studies is the importance of easy access to both the RSV vaccine and monoclonal antibody treatments for pregnant individuals and newborns. In California, 78% of the RSV vaccines were administered during prenatal visits, which helped streamline the process and reduce barriers to vaccination. Ensuring that the RSV vaccine is available at prenatal clinics and that monoclonal antibody treatments are available on labor and delivery units could further enhance the efficacy of vaccination campaigns. Simplifying access to these vital tools is crucial in making RSV prevention accessible to all at-risk populations.
Dr. Ai-ris Yonekura Collier, an assistant professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, co-authored another study on RSV vaccination. Her study, conducted at a Boston medical center, found lower uptake rates than the California study, with just under 21% of pregnant individuals opting for the vaccine and 35% of babies receiving monoclonal antibodies. Despite these lower numbers, the study highlighted the potential of achieving RSV herd immunity with increased vaccination rates.
Collier cited a study from Spain that achieved nearly 90% coverage for monoclonal antibody treatment, demonstrating real-world effectiveness in preventing RSV. The study’s results suggest that with increased uptake and availability of the RSV vaccine and monoclonal antibodies, it may be possible to stop the spread of RSV on a population level. While nationwide data suggests a lower uptake rate, the findings from both California and Boston underscore the importance of making these vaccines and treatments available in prenatal and delivery settings.
The Importance of Continued Research and Access
The breakthrough findings from the UCSF and Boston studies underscore the need for continued research and implementation of RSV prevention measures. With RSV being a leading cause of infant hospitalization and death, ensuring that vaccines and monoclonal antibodies are widely available and accessible is vital for the protection of infants worldwide. As Blauvelt and Collier emphasized, making vaccines and treatments available in OB/GYN offices and delivery units could significantly reduce barriers to vaccination.
While RSV vaccines for pregnant individuals are still relatively new, these studies provide crucial evidence of their safety and efficacy. As the data continues to emerge, it is important for healthcare providers and public health authorities to work together to increase vaccine uptake, reduce barriers to access, and protect vulnerable infants from RSV.
The latest studies offer a glimmer of hope in the fight against RSV, a virus that has caused so many infant hospitalizations and deaths worldwide. With the development of the RSV vaccine for pregnant individuals and the use of monoclonal antibodies for infants, the potential to protect newborns from this dangerous disease is within reach. However, continued research, increased vaccine uptake, and improved access to treatments are essential to ensure that RSV immunity is achieved on a population-wide scale. If these efforts are successful, the future of RSV prevention looks bright, and countless infants may be spared from the severe consequences of this disease.